OneContact
Digital Consultations
Improve access and efficiency by communicating remotely with patients when a face to face appointment is unnecessary.
OneContact supports both inbound and outbound patient communication, with flexible configurability so you can scale the extent of your digital communication options over time.

Outbound Contact
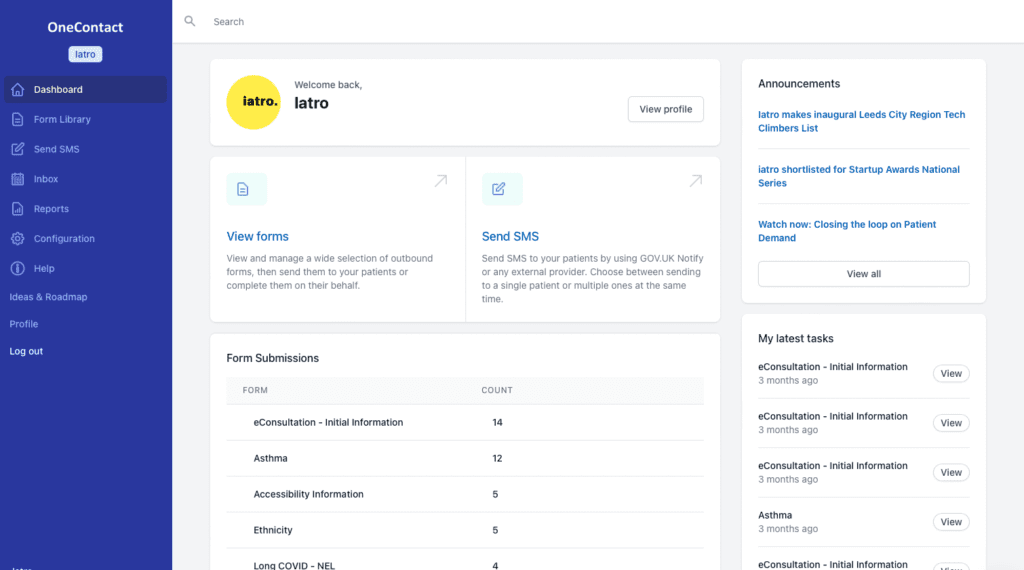
Conduct clinical reviews remotely with our wide range of clinical review templates for asthma, diabetes, hypertension, health checks and more.
Patients are sent a text link to a form which captures relevant QOF data alongside additional clinical information.
Clinicians can hone down on the most important areas quickly and only bring patients in for the elements of care that are essential.
Inbound Contact
OneContact’s video and e-consultation capabilities are easily embedded into GP practice websites, with rich, dynamic and clinically validated templates that triage and signpost patients to the most appropriate treatment or services.
Where a GP appointment is still necessary, the forms provide clinicians with a succinct overview of relevant clinical information in advance.
Managing Demand
OneContact puts you in control of your demand, with the ability to enable each form individually on its own schedule or once a threshold is met.

An ever expanding administration and clinical form library.

Accessibility Information
This form allows you to collect accessibility information from your patients such as commmunication needs, or a carer/parent/guardings communication needs.

Alcohol Use Disorders Identification Test For Consumption (AUDIT-C)
The Alcohol Use Disorders Identification Test (AUDIT-C) is an alcohol screen that can help identify patients who are hazardous drinkers or have active alcohol use disorders (including alcohol abuse or dependence)
Asthma Control Test
The Asthma Control Test provides a numerical score to help you and your healthcare provider determine if your asthma symptoms are well controlled.

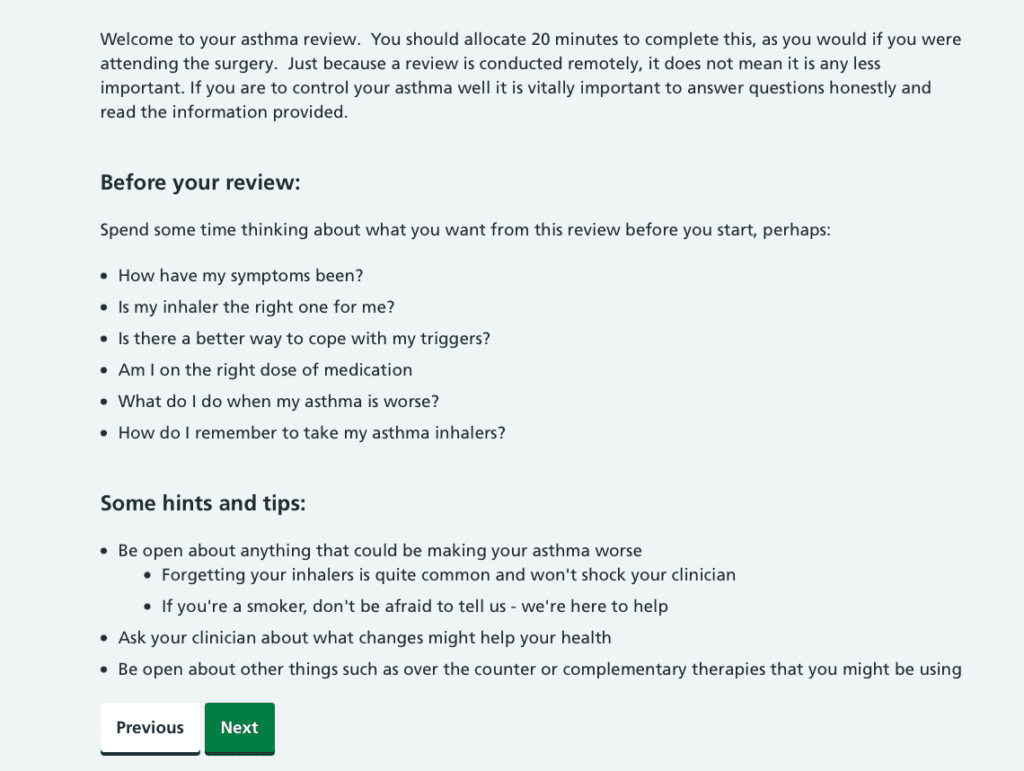
Asthma Review
A holistic and thorough remote review for asthma, incorporating NICE guidance. Patients can send videos of their inhaler technique and there are embedded videos for them to watch on the devices that they are using. This can allow your team to screen which patients are well controlled and don’t need a full review, and which need further input.

Care Home Data Collection

Carer Information
You can use this form to enquire about carers amongst your patients.

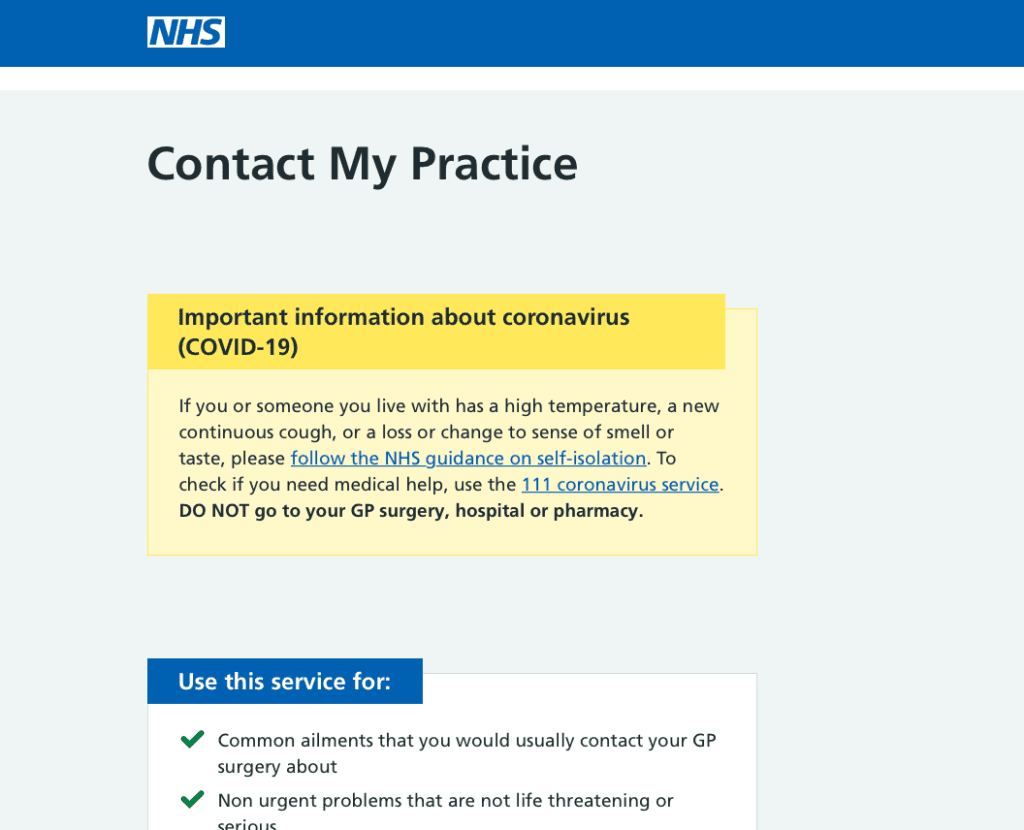
Contact My Practice
Triage and appointment request form for acute ‘on the day’ requests.

COVID Checkup (Oximetry & SATs monitoring)
This form supports the Oximetry@home programme to manage patients who have been diagnosed with COVID and are managing themselves at home, with an oximetry device

Diabetes Review
Every child and young person with diabetes is required to have an annual review once a year. The annual review provides an opportunity to take a look at all aspects of your diabetes, what is going well, what may not be going so well and to check for any early signs of other health concerns which may be related to it.

Diet and Activity
Patients can submit information about their diet and activity levels (GPAQ) using this form.

Flu Consent (Child)
This form can be used to obtain consent for flu vaccination in children prior to them attending.

Generalised Anxiety Disorder Assessment (GAD-7)
The Generalised Anxiety Disorder Assessment (GAD-7) is a seven-item instrument that is used to measure or assess the severity of generalised anxiety disorder (GAD). Each item asks the individual to rate the severity of his or her symptoms over the past two weeks.

Heart Failure Review
Developed in collaboration with colleagues in London this is a comprehensive review for patients suffering from heart failure. It takes account of symptoms of breathlessness, cough, fatigue and also screens for mental health issues.

Hypertension / Raised BP
This review allows patients to submit home blood measurements for review as well as conducting a thorough review for hypertensive patients, incorporating risk factor assessment. Patients can choose different durations of monitoring to submit and then return to the form each day to fill in their readings.

International Prostate Symptom Score (IPSS)
The American Urological Association (AUA) has developed the following questionnaire to help men determine how bothersome their urinary symptoms are and to check how effective their treatment is. This questionnaire has also been adopted worldwide and is known as the International Prostate Symptom Score (IPSS).

Learning Disabilities Annual Health Check
These annual health checks are for adults and young people aged 14 or over with a learning disability. An annual health check helps you stay well by talking about your health and finding any problems early, so you get the right care.

Smoking and Weight
You can use this form to collect details about patients smoking status and current height/weight/BMI.

Video Group Clinic Patient Consent
This form captures consent from patients for Redmoor’s video group clinic, alongside contact information for the session.
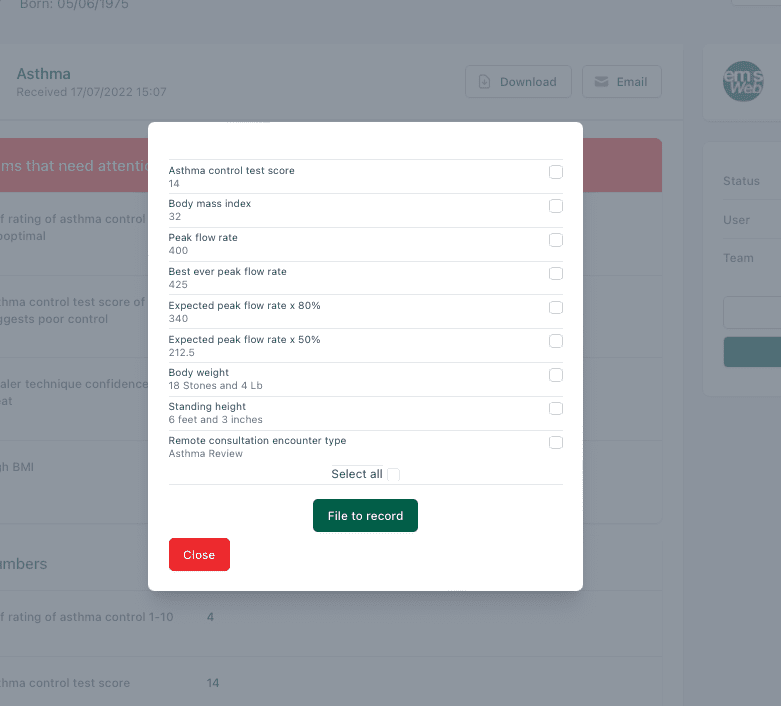
Clinical System Integration
01.
Find & Link
Using the information the patient has supplied OneContact links the patient to the record.
02.
Select Codes
Choose from a list of suggested SNOMED codes, pre-populated from the form.
03.
File
With one click, The chosen SNOMED codes are filed to the clinical system
04.
Attach
Optionally, you can attach a PDF of the full form to the record.
A partnership between clinical, software & telephony specialists

Primary Care IT are led by a team of clinicians who specialise in producing clinical content and tools to improve efficiencies across Primary Care.

iatro are a leading provider of software in Primary Care.

X-on are a market leading cloud telephony provider to the NHS.